In Giancoli section 18-1, it is derived that if the average kinetic energy of a gas molecule is denote by
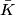 , ,  This implies that  Therefore, 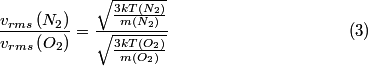 The temperatures for the two gases are assumed to be the same, because they are both in the same mixture. Therefore, 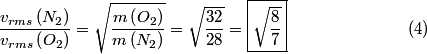 |